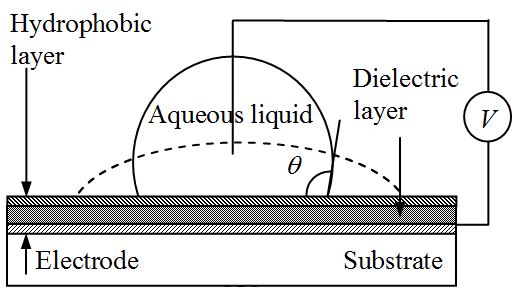

Figure illustrates the typical EWOD configuration with an aqueous sessile droplet resting on a dielectric layer covering the electrode underneath. When the electric voltage V is applied between the aqueous sessile droplet and the electrode, the droplet spontaneously spreads out on the dielectric surface. As a result, the contact angle which is defined in the liquid side is decreased. Note that the applied electric voltage (V) is valid for AC as well as DC voltages, and V is defined as the voltage across the dielectric layer, not between the electrodes. When the electric potential V is removed, the changed contact angle returns to the initial contact angle. That is, the droplet contacts back to the initial shape. As a result, the wettability of liquids on a dielectric surface can be electrically controlled with high reversibility. This phenomenon, called as electrowetting (to be exact, electrowetting on dielectric or EWOD), has outstanding features: (1) excellent reversibility; (2) extremely low power consumption (less than mW) since the dielectric layer acts like a capacitor, not energy dissipation devices; and (3) superb robustness since there is no direct electrochemical interaction between the electrode and liquid.
